Recombinant Human Carboxypeptidase A2/CPA2
| Product name: | Recombinant Human Carboxypeptidase A2/CPA2 |
| Source: | Human Cells |
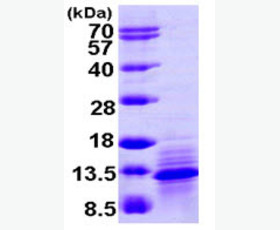
| Purity: | Greater than 95% as determined by reducing SDS-PAGE. |
| Buffer Formulation: | Lyophilized from a 0.2 μm filtered solution of 20mM TrisHCl, 150mm NaCl, pH 7.5. |
| Applications: | Applications:SDS-PAGE; WB; ELISA; IP. |
| Storage: | Avoid repeated freeze/thaw cycles. Store at 2-8 oC for one month. Aliquot and store at -80 oC for 12 months. |
| UOM: | 100ug/50ug/200ug/1mg/1g |
| Source | Human Cells |
| Description | Recombinant Human Carboxypeptidase A2 is produced by our Mammalian expression system and the target gene encoding Leu17-Tyr417 is expressed with a 6His tag at the C-terminus. |
| Names | Carboxypeptidase A2, CPA2 |
| Accession # | P48052 |
| Formulation | Lyophilized from a 0.2 μm filtered solution of 20mM TrisHCl, 150mm NaCl, pH 7.5. |
| Shipping |
The product is shipped at ambient temperature. |
| Reconstitution |
Always centrifuge tubes before opening. Do not mix by vortex or pipetting. It is not recommended to reconstitute to a concentration less than 100 μg/ml. Dissolve the lyophilized protein in ddH2O. Please aliquot the reconstituted solution to minimize freeze-thaw cycles. |
| Storage |
Lyophilized protein should be stored at < -20°C, though stable at room temperature for 3 weeks. Reconstituted protein solution can be stored at 4-7°C for 2-7 days. Aliquots of reconstituted samples are stable at < -20°C for 3 months. |
| Purity |
Greater than 95% as determined by reducing SDS-PAGE. |
| Endotoxin | Less than 0.1 ng/µg (1 IEU/µg) as determined by LAL test. |
| Amino Acid Sequence |
LETFVGDQVLEIVPSNEEQIKNLLQLEAQEHLQLDFWKSPTTPGETAHVRVPFVNVQAVKVFLGS QGIAYSIMIEDVQVLLDKENEEMLFNRRRERSGNFNFGAYHTLEEISQEMDNLVAEHPGLVSKVN IGSSFENRPMNVLKFSTGGDKPAIWLDAGIHAREWVTQATALWTANKIVSDYGKDPSITSILDAL DIFLLPVTNPDGYVFSQTKNRMWRKTRSKVSGSLCVGVDPNRNWDAGFGGPGASSNPCSDSYHGP SANSEVEVKSIVDFIKSHGKVKAFITLHSYSQLLMFPYGYKCTKLDDFDELSEVAQKAAQSLRSL HGTKYKVGPICSVIYQASGGSIDWSYDYGIKYSFAFELRDTGRYGFLLPARQILPTAEETWLGLK AIMEHVRDHPYVDHHHHHH
|
| Background | Carboxypeptidase A2 (CPA) is a secreted pancreatic procarboxy-peptidase that cleaves the C-terminal amide or ester bond of peptides that have a free C-terminal carboxyl group. The hydrolytic action of CPA2 was identified with a preference towards long substrates with aromatic amino acids in their C-terminal end, particularly tryptophan. CPA2 comprises a signal peptide, a pro region and a mature chain, and can be activated after cleavage of the pro peptide. Three different forms of human pancreatic procarboxypeptidase A have been isolated, and the A1 and A2 forms are always secreted as monomeric proteins with different biochemical properties. In contrast to procarboxypeptidase B which was always secreted by the pancreas as a monomer, procarboxypeptidase A occurs as a monomer and/or associated to one or two functionally different proteins, such as zymogen E, and is involved in zymogen inhibition. |