Recombinant E. coli Malate Dehydrogenase/MDH
| Product name: | Recombinant E. coli Malate Dehydrogenase/MDH |
| Source: | E.coli |
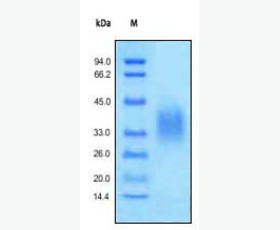
| Purity: | Greater than 95% as determined by reducing SDS-PAGE. |
| Buffer Formulation: | Supplied as a 0.2 μm filtered solution of 50mM PB,50%Glycerol,pH7.5. |
| Applications: | Applications:SDS-PAGE; WB; ELISA; IP. |
| Storage: | Avoid repeated freeze/thaw cycles. Store at 2-8 oC for one month. Aliquot and store at -80 oC for 12 months. |
| UOM: | 100ug/50ug/200ug/1mg/1g |
| Source | E.coli |
| Description | Recombinant E.coli Malate Dehydrogenase is produced by our E.coli expression system and the target gene encoding Met1-Lys312 is expressed with a 6His tag at the N-terminus. |
| Names | Malate dehydrogenase,mdh |
| Accession # | P61889 |
| Formulation | Supplied as a 0.2 μm filtered solution of 50mM PB,50%Glycerol,pH7.5. |
| Shipping |
The product is shipped on dry ice/ice packs. |
| Storage |
Store at < -20°C, stable for 6 months after receipt. Please minimize freeze-thaw cycles. |
| Purity |
Greater than 95% as determined by reducing SDS-PAGE. |
| Endotoxin | Less than 0.1 ng/µg (1 IEU/µg) as determined by LAL test. |
| Amino Acid Sequence |
MGSSHHHHHHSSGLVPRGSHMKVAVLGAAGGIGQALALLLKTQLPSGSELSLYDIAPVTPGVAVD LSHIPTAVKIKGFSGEDATPALEGADVVLISAGVRRKPGMDRSDLFNVNAGIVKNLVQQVAKTCP KACIGIITNPVNTTVAIAAEVLKKAGVYDKNKLLGVTTLDIIRSNTFVAELKGKQPGEVEVPVIG GHSGVTILPLLSQVPGVSFTEQEVADLTKRIQNAGTEVVEAKAGGGSATLSMGQAAARFGLSLVR ALQGEQGVVECAYVEGDGQYARFFSQPLLLGKNGVEERKSIGTLSAFEQNALEGMLDTLKKDIAL GQEFVNK
|
| Background | Escherichia coli MDH, also known as Malate dehydrogenase, is a group of multimeric enzymes consisting of identical subunits usually organized as either dimer or tetramers with subunit molecular weights of 30-35 kDa. Its major function is to catalyze the NAD / NADH-dependent interconversion of the substrates malate and oxaloacetate. This reaction plays a key part in the malate / aspartate shuttle across the mitochondrial membrane, and in the tricarboxylic acid cycle within the mitochondrial matrix.MDH is synthesized in several organisms including archaea, eubacteria, fungi, plant and mammals. The enzymes share a common catalytic mechanism and their kinetic properties are similar, which demonstrates a high degree of structural similarity. The three-dimensional structures and elements essential for catalysis are conserved between mitochondrial and cytoplasmic forms of MDH in eukaryotic cells even though these isoenzymes are only marginally related at the level of primary structure. |