Recombinant Human Coagulation Factor XIII A Chain
| Product name: | Recombinant Human Coagulation Factor XIII A Chain |
| Source: | Human Cells |
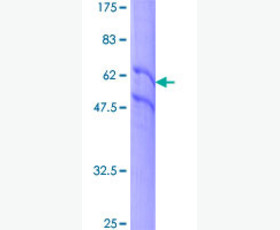
| Purity: | Greater than 95% as determined by reducing SDS-PAGE. |
| Buffer Formulation: | Supplied as a 0.2 μm filtered solution of 50 mM NaCl,5% Sucrose, 1% Tween 20 (v/v),0.3% Histidine (w/v),pH8.0. |
| Applications: | Applications:SDS-PAGE; WB; ELISA; IP. |
| Storage: | Avoid repeated freeze/thaw cycles. Store at 2-8 oC for one month. Aliquot and store at -80 oC for 12 months. |
| UOM: | 100ug/50ug/200ug/1mg/1g |
SourceHuman CellsDescriptionRecombinant Human Coagulation Factor XIII A Chain is produced by our Mammalian expression system and the target gene encoding Gly39-Met732 is expressed with a 6His tag at the C-terminus.NamesCoagulation Factor XIII A Chain; Coagulation Factor XIIIa; Protein-Glutamine Gamma-Glutamyltransferase A Chain; Transglutaminase A Chain; F13A1; F13AAccession #P00488FormulationSupplied as a 0.2 μm filtered solution of 50 mM NaCl,5% Sucrose, 1% Tween 20 (v/v),0.3% Histidine (w/v),pH8.0.ShippingThe product is shipped on dry ice/ice packs.
StorageStore at < -20°C, stable for 6 months after receipt.
Please minimize freeze-thaw cycles.PurityGreater than 95% as determined by reducing SDS-PAGE.
EndotoxinLess than 0.1 ng/µg (1 IEU/µg) as determined by LAL test.Amino Acid Sequence
StorageStore at < -20°C, stable for 6 months after receipt.
Please minimize freeze-thaw cycles.PurityGreater than 95% as determined by reducing SDS-PAGE.
EndotoxinLess than 0.1 ng/µg (1 IEU/µg) as determined by LAL test.Amino Acid Sequence
GVNLQEFLNVTSVHLFKERWDTNKVDHHTDKYENNKLIVRRGQSFYVQIDFSRPYDPRRDLFRVE YVIGRYPQENKGTYIPVPIVSELQSGKWGAKIVMREDRSVRLSIQSSPKCIVGKFRMYVAVWTPY GVLRTSRNPETDTYILFNPWCEDDAVYLDNEKEREEYVLNDIGVIFYGEVNDIKTRSWSYGQFED GILDTCLYVMDRAQMDLSGRGNPIKVSRVGSAMVNAKDDEGVLVGSWDNIYAYGVPPSAWTGSVD ILLEYRSSENPVRYGQCWVFAGVFNTFLRCLGIPARIVTNYFSAHDNDANLQMDIFLEEDGNVNS KLTKDSVWNYHCWNEAWMTRPDLPVGFGGWQAVDSTPQENSDGMYRCGPASVQAIKHGHVCFQFD APFVFAEVNSDLIYITAKKDGTHVVENVDATHIGKLIVTKQIGGDGMMDITDTYKFQEGQEEERL ALETALMYGAKKPLNTEGVMKSRSNVDMDFEVENAVLGKDFKLSITFRNNSHNRYTITAYLSANI TFYTGVPKAEFKKETFDVTLEPLSFKKEAVLIQAGEYMGQLLEQASLHFFVTARINETRDVLAKQ KSTVLTIPEIIIKVRGTQVVGSDMTVTVQFTNPLKETLRNVWVHLDGPGVTRPMKKMFREIRPNS TVQWEEVCRPWVSGHRKLIASMSSDSLRHVYGELDVQIQRRPSMVDHHHHHH
BackgroundCoagulation factor XIII is the last zymogen to become activated in the blood coagulation cascade. Plasma factor XIII is a heterotetramer composed of 2 A subunits and 2 B subunits. The A subunits have catalytic function, and the B subunits do not have enzymatic activity and may serve as plasma carrier molecules. Platelet factor XIII is composed of just 2 A subunits, which are identical to those of plasma origin. Upon cleavage of the activation peptide by thrombin and in the presence of calcium ion, the plasma factor XIII dissociates its B subunits and yields the same active enzyme, factor XIIIa, as platelet factor XIII. This enzyme acts as a transglutaminase to catalyze the formation of gamma-glutamyl-epsilon-lysine crosslinking between fibrin molecules, thus stabilizing the fibrin clot. Factor XIII deficiency is classified into two categories: type I deficiency, characterized by the lack of both the A and B subunits; and type II deficiency, characterized by the lack of the A subunit alone. These defects can result in a lifelong bleeding tendency, defective wound healing, and habitual abortion.