Recombinant Human Myeloperoxidase/MPO
| Product name: | Recombinant Human Myeloperoxidase/MPO |
| Source: | Human Cells |
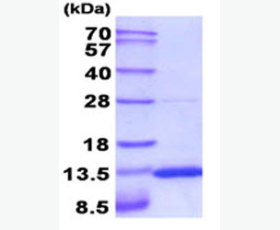
| Purity: | Greater than 95% as determined by reducing SDS-PAGE. |
| Buffer Formulation: | Lyophilized from a 0.2 μm filtered solution of 20mM Tris, 150mM NaCl, pH8.0 . |
| Applications: | Applications:SDS-PAGE; WB; ELISA; IP. |
| Storage: | Avoid repeated freeze/thaw cycles. Store at 2-8 oC for one month. Aliquot and store at -80 oC for 12 months. |
| UOM: | 100ug/50ug/200ug/1mg/1g |
| Source | Human Cells |
| Description | Recombinant Human Myeloperoxidase is produced by our Mammalian expression system and the target gene encoding Ala49-Ser745 is expressed with a 10His tag at the C-terminus. |
| Names | Myeloperoxidase; MPO |
| Accession # | P05164 |
| Formulation | Lyophilized from a 0.2 μm filtered solution of 20mM Tris, 150mM NaCl, pH8.0 . |
| Shipping |
The product is shipped at ambient temperature. |
| Reconstitution |
Always centrifuge tubes before opening. Do not mix by vortex or pipetting. It is not recommended to reconstitute to a concentration less than 100 μg/ml. Dissolve the lyophilized protein in ddH2O. Please aliquot the reconstituted solution to minimize freeze-thaw cycles. |
| Storage |
Lyophilized protein should be stored at < -20°C, though stable at room temperature for 3 weeks. Reconstituted protein solution can be stored at 4-7°C for 2-7 days. Aliquots of reconstituted samples are stable at < -20°C for 3 months. |
| Purity |
Greater than 95% as determined by reducing SDS-PAGE. |
| Endotoxin | Less than 0.1 ng/µg (1 IEU/µg) as determined by LAL test. |
| Amino Acid Sequence |
AAPAVLGEVDTSLVLSSMEEAKQLVDKAYKERRESIKQRLRSGSASPMELLSYFKQPVAATRTAV RAADYLHVALDLLERKLRSLWRRPFNVTDVLTPAQLNVLSKSSGCAYQDVGVTCPEQDKYRTITG MCNNRRSPTLGASNRAFVRWLPAEYEDGFSLPYGWTPGVKRNGFPVALARAVSNEIVRFPTDQLT PDQERSLMFMQWGQLLDHDLDFTPEPAARASFVTGVNCETSCVQQPPCFPLKIPPNDPRIKNQAD CIPFFRSCPACPGSNITIRNQINALTSFVDASMVYGSEEPLARNLRNMSNQLGLLAVNQRFQDNG RALLPFDNLHDDPCLLTNRSARIPCFLAGDTRSSEMPELTSMHTLLLREHNRLATELKSLNPRWD GERLYQEARKIVGAMVQIITYRDYLPLVLGPTAMRKYLPTYRSYNDSVDPRIANVFTNAFRYGHT LIQPFMFRLDNRYQPMEPNPRVPLSRVFFASWRVVLEGGIDPILRGLMATPAKLNRQNQIAVDEI RERLFEQVMRIGLDLPALNMQRSRDHGLPGYNAWRRFCGLPQPETVGQLGTVLRNLKLARKLMEQ YGTPNNIDIWMGGVSEPLKRKGRVGPLLACIIGTQFRKLRDGDRFWWENEGVFSMQQRQALAQIS LPRIICDNTGITTVSKNNIFMSNSYPRDFVNCSTLPALNLASWREASHHHHHHHHHH
|
| Background | Myeloperoxidase (MPO) is a heme-containing enzyme belonging to the XPO subfamily of peroxidases. It is an abundant neutrophil and monocyte glycoprotein that catalyzes the hydrogen peroxide-dependent conversion of chloride, bromide, and iodide to multiple reactive species. Post-translational processing of MPO involves the insertion of a heme moiety and the proteolytic removal of both a propeptide and a 6 aa internal peptide. This results in a disulfide-linked dimer composed of a 60 kDa heavy and 12 kDa light chain that associate into a 150 kDa enzymatically active tetramer. The tetramer contains two heme groups and one disulfide bond between the heavy chains. Alternate splicing generates two additional isoforms of MPO, one with a 32 aa insertion in the light chain, and another with a deletion of the signal sequence and part of the propeptide. Human and mouse MPO share 87% aa sequence identity. MPO activity results in protein nitrosylation and the formation of 3-chlorotyrosine and dityrosine crosslinks. MPO is also associated with a variety of other diseases, and inhibits vasodilation in inflammation by depleting the levels of NO. Serum albumin functions as a carrier protein during MPO movement to the basolateral side of epithelial cells. MPO is stored in neutrophil azurophilic granules. Upon cellular activation, it is deposited into pathogen-containing phagosomes. |