Recombinant Human Calreticulin-3/CALR3/CRT2
| Product name: | Recombinant Human Calreticulin-3/CALR3/CRT2 |
| Source: | E. coli |
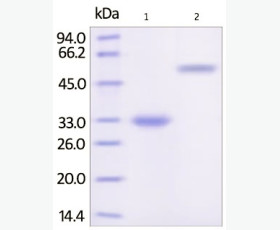
| Purity: | Greater than 95% as determined by reducing SDS-PAGE. |
| Buffer Formulation: | Lyophilized from a 0.2 μm filtered solution of 20mM PB,150mM NaCl,pH7.4. |
| Applications: | Applications:SDS-PAGE; WB; ELISA; IP. |
| Storage: | Avoid repeated freeze/thaw cycles. Store at 2-8 oC for one month. Aliquot and store at -80 oC for 12 months. |
| UOM: | 100ug/50ug/200ug/1mg/1g |
| Source | E. coli |
| Description | Recombinant Human calreticulin-3 is produced by our E.coli expression system and the target gene encoding Thr20-Leu384 is expressed. |
| Names | Calreticulin-3, calreticulin-2, calsperin, CALR3, CRT2 |
| Accession # | Q96L12 |
| Formulation | Lyophilized from a 0.2 μm filtered solution of 20mM PB,150mM NaCl,pH7.4. |
| Shipping |
The product is shipped at ambient temperature. |
| Reconstitution |
Always centrifuge tubes before opening. Do not mix by vortex or pipetting. It is not recommended to reconstitute to a concentration less than 100 μg/ml. Dissolve the lyophilized protein in ddH2O. Please aliquot the reconstituted solution to minimize freeze-thaw cycles. |
| Storage |
Lyophilized protein should be stored at < -20°C, though stable at room temperature for 3 weeks. Reconstituted protein solution can be stored at 4-7°C for 2-7 days. Aliquots of reconstituted samples are stable at < -20°C for 3 months. |
| Purity |
Greater than 95% as determined by reducing SDS-PAGE. |
| Endotoxin | Less than 0.1 ng/µg (1 IEU/µg) as determined by LAL test. |
| Amino Acid Sequence |
MTVYFQEEFLDGEHWRNRWLQSTNDSRFGHFRLSSGKFYGHKEKDKGLQTTQNGRFYAISARFKP FSNKGKTLVIQYTVKHEQKMDCGGGYIKVFPADIDQKNLNGKSQYYIMFGPDICGFDIKKVHVIL HFKNKYHENKKLIRCKVDGFTHLYTLILRPDLSYDVKIDGQSIESGSIEYDWNLTSLKKETSPAE SKDWEQTKDNKAQDWEKHFLDASTSKQSDWNGDLDGDWPAPMLQKPPYQDGLKPEGIHKDVWLHR KMKNTDYLTQYDLSEFENIGAIGLELWQVRSGTIFDNFLITDDEEYADNFGKATWGETKGPEREM DAIQAKEEMKKAREEEEEELLSGKINRHEHYFNQFHRRNEL
|
| Background | Calreticulin-3 belongs to the calreticulin family, members of which are calcium binding chaperones localized mainly in the endoplasmic reticulum. It can be divided into a N-terminal globular domain, a proline-rich P-domain forming an elongated arm-like structure and a C-terminal acidic domain. During spermatogenesis process, Calreticulin-3 may act as a lectin-independent chaperone for specific client proteins such as ADAM3. Defects in CALR3 are the cause of familial hypertrophic cardiomyopathy type 19 (CMH19), it is a hereditary heart disorder characterized by ventricular hypertrophy, which is usually asymmetric and often involves the interventricular septum. The symptoms include dyspnea, syncope, collapse, palpitations, and chest pain. |