Recombinant Human UDP-Glucose 4-Epimerase/GALE
| Product name: | Recombinant Human UDP-Glucose 4-Epimerase/GALE |
| Source: | E.coli |
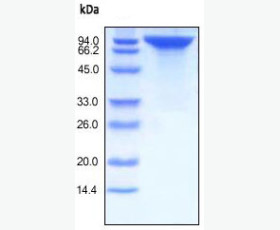
| Purity: | Greater than 95% as determined by reducing SDS-PAGE. |
| Buffer Formulation: | Supplied as a 0.2 μm filtered solution of 50mM TrisHCl, 150mM NaCl, 2mM DTT, 1mM EDTA, pH 8.0. |
| Applications: | Applications:SDS-PAGE; WB; ELISA; IP. |
| Storage: | Avoid repeated freeze/thaw cycles. Store at 2-8 oC for one month. Aliquot and store at -80 oC for 12 months. |
| UOM: | 100ug/50ug/200ug/1mg/1g |
| Source | E.coli |
| Description | Recombinant Human GALE is produced by our E.coli expression system and the target gene encoding Met1-Ala348 is expressed with a 6His tag at the N-terminus. |
| Names | UDP-Glucose 4-Epimerase, Galactowaldenase, UDP-Galactose 4-Epimerase, GALE |
| Accession # | Q14376 |
| Formulation | Supplied as a 0.2 μm filtered solution of 50mM TrisHCl, 150mM NaCl, 2mM DTT, 1mM EDTA, pH 8.0. |
| Shipping |
The product is shipped on dry ice/ice packs. |
| Storage |
Store at < -20°C, stable for 6 months after receipt. Please minimize freeze-thaw cycles. |
| Purity |
Greater than 95% as determined by reducing SDS-PAGE. |
| Endotoxin | Less than 0.1 ng/µg (1 IEU/µg) as determined by LAL test. |
| Amino Acid Sequence |
MGSSHHHHHHSSGLVPRGSHMAEKVLVTGGAGYIGSHTVLELLEAGYLPVVIDNFHNAFRGGGSL PESLRRVQELTGRSVEFEEMDILDQGALQRLFKKYSFMAVIHFAGLKAVGESVQKPLDYYRVNLT GTIQLLEIMKAHGVKNLVFSSSATVYGNPQYLPLDEAHPTGGCTNPYGKSKFFIEEMIRDLCQAD KTWNAVLLRYFNPTGAHASGCIGEDPQGIPNNLMPYVSQVAIGRREALNVFGNDYDTEDGTGVRD YIHVVDLAKGHIAALRKLKEQCGCRIYNLGTGTGYSVLQMVQAMEKASGKKIPYKVVARREGDVA ACYANPSLAQEELGWTAALGLDRMCEDLWRWQKQNPSGFGTQA
|
| Background | The enzyme UDP-Glucose 4-Epimerase (GALE) is a homodimeric epimerase found in bacterial, plant and mammalian cells. UDP-Glucose 4-Epimerase performs the final step in the Leloir pathway of Galactose metabolism, it catalyzes two distinct but analogous reactions: the epimerization of UDP-Gglucose to UDP-Galactose and the epimerization of UDP-N-Acetylglucosamine to UDP-N-Acetylgalactosamine. The bifunctional nature of the enzyme has the important metabolic consequence that mutant cells (or individuals) are dependent not only on exogenous galactose, but also on exogenous N-acetylgalactosamine as a necessary precursor for the synthesis of glycoproteins and glycolipids. |