Recombinant Human Major Prion Protein/PRP/CD230
| Product name: | Recombinant Human Major Prion Protein/PRP/CD230 |
| Source: | E.coli |
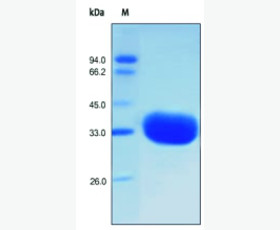
| Purity: | Greater than 95% as determined by reducing SDS-PAGE. |
| Buffer Formulation: | Lyophilized from a 0.2 μm filtered solution of 5mM PB, 200mM NaCl, pH 7.5. |
| Applications: | Applications:SDS-PAGE; WB; ELISA; IP. |
| Storage: | Avoid repeated freeze/thaw cycles. Store at 2-8 oC for one month. Aliquot and store at -80 oC for 12 months. |
| UOM: | 100ug/50ug/200ug/1mg/1g |
| Source | E.coli |
| Description | Recombinant Human Major Prion Protein is produced by our E.coli expression system and the target gene encoding Gln91-Ser231 is expressed. |
| Names | Major Prion Protein, PrP, ASCR, PrP27-30, PrP33-35C, CD230, PRNP, PRIP, PRP |
| Accession # | P04156 |
| Formulation | Lyophilized from a 0.2 μm filtered solution of 5mM PB, 200mM NaCl, pH 7.5. |
| Shipping |
The product is shipped at ambient temperature. |
| Reconstitution |
Always centrifuge tubes before opening. Do not mix by vortex or pipetting. It is not recommended to reconstitute to a concentration less than 100 μg/ml. Dissolve the lyophilized protein in ddH2O. Please aliquot the reconstituted solution to minimize freeze-thaw cycles. |
| Storage |
Lyophilized protein should be stored at < -20°C, though stable at room temperature for 3 weeks. Reconstituted protein solution can be stored at 4-7°C for 2-7 days. Aliquots of reconstituted samples are stable at < -20°C for 3 months. |
| Purity |
Greater than 95% as determined by reducing SDS-PAGE. |
| Endotoxin | Less than 0.1 ng/µg (1 IEU/µg) as determined by LAL test. |
| Amino Acid Sequence |
MGQGGGTHSQWNKPSKPKTNMKHMAGAAAAGAVVGGLGGYVLGSAMSRPIIHFGSDYEDRYYREN MHRYPNQVYYRPMDEYSNQNNFVHDCVNITIKQHTVTTTTKGENFTETDVKMMERVVEQMCITQY ERESQAYYQRGSS
|
| Background | Major Prion Protein is unique in its ability to reproduce on its own and become infectious. The discovery of prion proteins as infectious agents began in the 1980s with an outbreak of mad cow disease in the United Kingdom. They are found in high quantity in the brain of humans and animals infected with neurodegenerative diseases known as transmissible spongiform encephalopathies or prion diseases. They can occur in two forms called PrP-sen and PrP-res. The normal, monomeric form has a mainly alpha-helical structure. The disease-associated, protease-resistant form forms amyloid fibrils containing a cross-beta spine, formed by a steric zipper of superposed beta-strands. Disease mutations may favor intermolecular contacts via short beta strands, and may thereby trigger oligomerization. Contains an N-terminal region composed of octamer repeats. Diseases caused by prions are known as spongiform diseases, because the brain tissue in infected individuals is filled with holes, giving it a sponge-like appearance. Although prions are found throughout the brain, the symptoms of spongiform diseases vary according to the regions. There are currently no effective treatments for spongiform diseases and all are fatal. Prions cannot be destroyed by boiling, alcohol, acid, standard autoclaving methods, or radiation. In fact, infected brains that have been sitting in formaldehyde for decades can still transmit spongiform disease. |