Recombinant Human Serpin E1/PAI-1
| Product name: | Recombinant Human Serpin E1/PAI-1 |
| Source: | Human Cells |
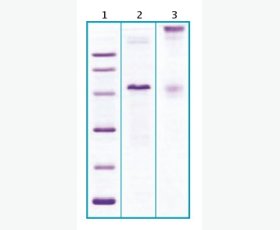
| Purity: | Greater than 95% as determined by reducing SDS-PAGE. |
| Buffer Formulation: | Lyophilized from a 0.2 μm filtered solution of 20mM HAc-NaAc, 150mM NaCl, pH 4.0. |
| Applications: | Applications:SDS-PAGE; WB; ELISA; IP. |
| Storage: | Avoid repeated freeze/thaw cycles. Store at 2-8 oC for one month. Aliquot and store at -80 oC for 12 months. |
| UOM: | 100ug/50ug/200ug/1mg/1g |
| Source | Human Cells |
| Description | Recombinant Human Serpin E1/PAI-1 is produced by our Mammalian expression system and the target gene encoding Val24-Pro402 is expressed with a 6His tag at the C-terminus. |
| Names | Plasminogen Activator Inhibitor 1, PAI, PAI-1, Endothelial Plasminogen Activator Inhibitor, Serpin E1, SERPINE1, PAI1, PLANH1 |
| Accession # | P05121 |
| Formulation | Lyophilized from a 0.2 μm filtered solution of 20mM HAc-NaAc, 150mM NaCl, pH 4.0. |
| Shipping |
The product is shipped at ambient temperature. |
| Reconstitution |
Always centrifuge tubes before opening. Do not mix by vortex or pipetting. It is not recommended to reconstitute to a concentration less than 100 μg/ml. Dissolve the lyophilized protein in ddH2O. Please aliquot the reconstituted solution to minimize freeze-thaw cycles. |
| Storage |
Lyophilized protein should be stored at < -20°C, though stable at room temperature for 3 weeks. Reconstituted protein solution can be stored at 4-7°C for 2-7 days. Aliquots of reconstituted samples are stable at < -20°C for 3 months. |
| Purity |
Greater than 95% as determined by reducing SDS-PAGE. |
| Endotoxin | Less than 0.1 ng/µg (1 IEU/µg) as determined by LAL test. |
| Amino Acid Sequence |
VHHPPSYVAHLASDFGVRVFQQVAQASKDRNVVFSPYGVASVLAMLQLTTGGETQQQIQAAMGFK IDDKGMAPALRHLYKELMGPWNKDEISTTDAIFVQRDLKLVQGFMPHFFRLFRSTVKQVDFSEVE RARFIINDWVKTHTKGMISNLLGKGAVDQLTRLVLVNALYFNGQWKTPFPDSSTHRRLFHKSDGS TVSVPMMAQTNKFNYTEFTTPDGHYYDILELPYHGDTLSMFIAAPYEKEVPLSALTNILSAQLIS HWKGNMTRLPRLLVLPKFSLETEVDLRKPLENLGMTDMFRQFQADFTSLSDQEPLHVAQALQKVK IEVNESGTVASSSTAVIVSARMAPEEIIMDRPFLFVVRHNPTGTVLFMGQVMEPLDHHHHHH
|
| Background | Serpins are a group of proteins with similar structures that were first identified as a set of proteins able to inhibit proteases. They are the largest and most diverse family of serine protease inhibitors which are involved in a number of fundamental biological processes such as blood coagulation, complement activation, fibrinolysis, angiogenesis, inflammation and tumor suppression and are expressed in a cell-specific manner. Serpin E1 is a secreted protein which belongs to the Serpin family. Serpin E1 acts as 'bait' for tissue plasminogen activator, urokinase, and protein C. Its rapid interaction with TPA may function as a major control point in the regulation of fibrinolysis. Defects in SERPINE1 are characterized by abnormal bleeding due to Serpin E1 defect in the plasma. High concentrations of Serpin E1 have been associated with thrombophilia which is an autosomal dominant disorder in which affected individuals are prone to develop serious spontaneous thrombosis. |