Recombinant Human Serpin F1/PEDF
| Product name: | Recombinant Human Serpin F1/PEDF |
| Source: | Human Cells |
| Purity: | Greater than 95% as determined by reducing SDS-PAGE. |
| Buffer Formulation: | Lyophilized from a 0.2 μm filtered solution of 20mM TrisHCl, 150mM NaCl, pH 8.0. |
| Applications: | Applications:SDS-PAGE; WB; ELISA; IP. |
| Storage: | Avoid repeated freeze/thaw cycles. Store at 2-8 oC for one month. Aliquot and store at -80 oC for 12 months. |
| UOM: | 100ug/50ug/200ug/1mg/1g |
| Source | Human Cells |
| Description | Recombinant Human Serpin F1/PEDF is produced by our Mammalian expression system and the target gene encoding Gln20-Pro418 is expressed with a 6His tag at the C-terminus. |
| Names | Pigment Epithelium-Derived Factor, PEDF, Cell Proliferation-Inducing Gene 35 Protein, EPC-1, Serpin F1, SERPINF1, PEDF |
| Accession # | P36955 |
| Formulation | Lyophilized from a 0.2 μm filtered solution of 20mM TrisHCl, 150mM NaCl, pH 8.0. |
| Shipping |
The product is shipped at ambient temperature. |
| Reconstitution |
Always centrifuge tubes before opening. Do not mix by vortex or pipetting. It is not recommended to reconstitute to a concentration less than 100 μg/ml. Dissolve the lyophilized protein in ddH2O. Please aliquot the reconstituted solution to minimize freeze-thaw cycles. |
| Storage |
Lyophilized protein should be stored at < -20°C, though stable at room temperature for 3 weeks. Reconstituted protein solution can be stored at 4-7°C for 2-7 days. Aliquots of reconstituted samples are stable at < -20°C for 3 months. |
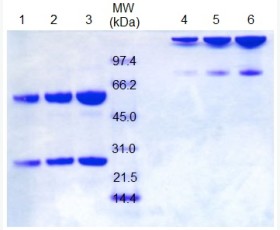
| Purity |
Greater than 95% as determined by reducing SDS-PAGE. |
| Endotoxin | Less than 0.1 ng/µg (1 IEU/µg) as determined by LAL test. |
| Amino Acid Sequence |
QNPASPPEEGSPDPDSTGALVEEEDPFFKVPVNKLAAAVSNFGYDLYRVRSSTSPTTNVLLSPLS VATALSALSLGAEQRTESIIHRALYYDLISSPDIHGTYKELLDTVTAPQKNLKSASRIVFEKKLR IKSSFVAPLEKSYGTRPRVLTGNPRLDLQEINNWVQAQMKGKLARSTKEIPDEISILLLGVAHFK GQWVTKFDSRKTSLEDFYLDEERTVRVPMMSDPKAVLRYGLDSDLSCKIAQLPLTGSMSIIFFLP LKVTQNLTLIEESLTSEFIHDIDRELKTVQAVLTVPKLKLSYEGEVTKSLQEMKLQSLFDSPDFS KITGKPIKLTQVEHRAGFEWNEDGAGTTPSPGLQPAHLTFPLDYHLNQPFIFVLRDTDTGALLFI GKILDPRGPVDHHHHHH
|
| Background | Serpin F1 is a secreted glycoprotein that belongs to the noninhibitory serpin. It has an alpha/beta core serine-protease inhibitor domain, three major beta-sheets, and ten alpha-helices. As protease inhibitors, serpins have an array of functions including regulating blood clotting, the complement pathway, extracellular matrix remodeling, and cell motility. They are also involved in activities that extend beyond their ability to inhibit proteases. For instance, they may also regulate blood pressure, angiogenesis, or act as storage/transport proteins. Serpin F1 is a new promising approach for the treatment of osteosarcoma and has been described as a natural angiogenesis inhibitor with neurotrophic and immune-modulation properties. The human serpin superfamily consists of at least 35 members that target not only serine proteases, but also selected cysteine proteases and non-protease proteins. Levels of the natural ocular anti-angiogenic factor SentrinF1 (PEDF) is associated with proliferative retinopathy. |