Recombinant Human Serpin G1/C1 Inhibitor
| Product name: | Recombinant Human Serpin G1/C1 Inhibitor |
| Source: | Human Cells |
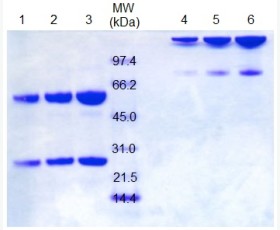
| Purity: | Greater than 95% as determined by reducing SDS-PAGE. |
| Buffer Formulation: | Lyophilized from a 0.2 μm filtered solution of 20mM TrisHCl, 150mM NaCl, pH 8.0. |
| Applications: | Applications:SDS-PAGE; WB; ELISA; IP. |
| Storage: | Avoid repeated freeze/thaw cycles. Store at 2-8 oC for one month. Aliquot and store at -80 oC for 12 months. |
| UOM: | 100ug/50ug/200ug/1mg/1g |
| Source | Human Cells |
| Description | Recombinant Human Serpin G1 is produced by our Mammalian expression system and the target gene encoding Asn23-Ala500 is expressed with a 6His tag at the C-terminus. |
| Names | Plasma Protease C1 Inhibitor, C1 Inh, C1Inh, C1 Esterase Inhibitor, C1-Inhibiting Factor, Serpin G1, SERPING1, C1IN, C1NH |
| Accession # | P05155 |
| Formulation | Lyophilized from a 0.2 μm filtered solution of 20mM TrisHCl, 150mM NaCl, pH 8.0. |
| Shipping |
The product is shipped at ambient temperature. |
| Reconstitution |
Always centrifuge tubes before opening. Do not mix by vortex or pipetting. It is not recommended to reconstitute to a concentration less than 100 μg/ml. Dissolve the lyophilized protein in ddH2O. Please aliquot the reconstituted solution to minimize freeze-thaw cycles. |
| Storage |
Lyophilized protein should be stored at < -20°C, though stable at room temperature for 3 weeks. Reconstituted protein solution can be stored at 4-7°C for 2-7 days. Aliquots of reconstituted samples are stable at < -20°C for 3 months. |
| Purity |
Greater than 95% as determined by reducing SDS-PAGE. |
| Endotoxin | Less than 0.1 ng/µg (1 IEU/µg) as determined by LAL test. |
| Amino Acid Sequence |
NPNATSSSSQDPESLQDRGEGKVATTVISKMLFVEPILEVSSLPTTNSTTNSATKITANTTDEPT TQPTTEPTTQPTIQPTQPTTQLPTDSPTQPTTGSFCPGPVTLCSDLESHSTEAVLGDALVDFSLK LYHAFSAMKKVETNMAFSPFSIASLLTQVLLGAGENTKTNLESILSYPKDFTCVHQALKGFTTKG VTSVSQIFHSPDLAIRDTFVNASRTLYSSSPRVLSNNSDANLELINTWVAKNTNNKISRLLDSLP SDTRLVLLNAIYLSAKWKTTFDPKKTRMEPFHFKNSVIKVPMMNSKKYPVAHFIDQTLKAKVGQL QLSHNLSLVILVPQNLKHRLEDMEQALSPSVFKAIMEKLEMSKFQPTLLTLPRIKVTTSQDMLSI MEKLEFFDFSYDLNLCGLTEDPDLQVSAMQHQTVLELTETGVEAAAASAISVARTLLVFEVQQPF LFMLWDQQHKFPVFMGRVYDPRAVDHHHHHH
|
| Background | The Human Serpin superfamily consists of at least 35 members that target not only serine proteases, but also selected cysteine proteases and non-protease proteins. As protease inhibitors, serpins have an array of functions including regulating blood clotting, the complement pathway, extracellular matrix remodeling, and cell motility. Serpin G1 is a serine protease inhibitor protein. It is the largest member among the serpin class of proteins. Remarkably, Serpin G1 has a 2-domain structure, unlike most family members. The C-terminal serpin domain is similar to other serpins, and this part of Serpin G1 provides the inhibitory activity. The N-terminal domain is not essential for Serpin G1 to inhibit proteinases and has no similarity to other proteins. The main function of Serpin G1 is the inhibition of the complement system to prevent spontaneous activation. Serpin G1 is an acute phase protein and circulates in blood at levels of around 0.25g/L, whose levels rise 2-fold during inflammation. Although named after its complement inhibitory activity, Serpin G1 also inhibits proteinases of the fibrinolytic, clotting, and kinin pathways. Most notably, Serpin G1 play a potentially crucial role in regulating important physiological pathways including complement activation, blood coagulation, fibrinolysis and the generation of kinins. It is also the most important physiological inhibitor of fXIIa, chymotrypsin and plasma kallikrein. |