Recombinant E. coli Tryptophan Synthase α Chain/Trp A
| Product name: | Recombinant E. coli Tryptophan Synthase α Chain/Trp A |
| Source: | E.coli |
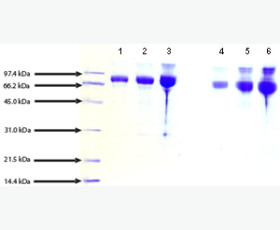
| Purity: | Greater than 95% as determined by reducing SDS-PAGE. |
| Buffer Formulation: | Lyophilized from a 0.2 μm filtered solution of PBS,pH7.4. |
| Applications: | Applications:SDS-PAGE; WB; ELISA; IP. |
| Storage: | Avoid repeated freeze/thaw cycles. Store at 2-8 oC for one month. Aliquot and store at -80 oC for 12 months. |
| UOM: | 100ug/50ug/200ug/1mg/1g |
| Source | E.coli |
| Description | Recombinant E.coli Tryptophan synthase alpha chain is produced by our E.coli expression system and the target gene encoding Met1-Ser268 is expressed. |
| Names | Tryptophan synthase alpha chain, trpA, |
| Accession # | P0A877 |
| Formulation | Lyophilized from a 0.2 μm filtered solution of PBS,pH7.4. |
| Shipping |
The product is shipped at ambient temperature. |
| Reconstitution |
Always centrifuge tubes before opening. Do not mix by vortex or pipetting. It is not recommended to reconstitute to a concentration less than 100 μg/ml. Dissolve the lyophilized protein in ddH2O. Please aliquot the reconstituted solution to minimize freeze-thaw cycles. |
| Storage |
Lyophilized protein should be stored at < -20°C, though stable at room temperature for 3 weeks. Reconstituted protein solution can be stored at 4-7°C for 2-7 days. Aliquots of reconstituted samples are stable at < -20°C for 3 months. |
| Purity |
Greater than 95% as determined by reducing SDS-PAGE. |
| Endotoxin | Less than 0.1 ng/µg (1 IEU/µg) as determined by LAL test. |
| Amino Acid Sequence |
MERYESLFAQLKERKEGAFVPFVTLGDPGIEQSLKIIDTLIEAGADALELGIPFSDPLADGPTIQ NATLRAFAAGVTPAQCFEMLALIRQKHPTIPIGLLMYANLVFNKGIDEFYAQCEKVGVDSVLVAD VPVEESAPFRQAALRHNVAPIFICPPNADDDLLRQIASYGRGYTYLLSRAGVTGAENRAALPLNH LVAKLKEYNAAPPLQGFGISAPDQVKAAIDAGAAGAISGSAIVKIIEQHINEPEKMLAALKVFVQ PMKAATRS
|
| Background | Tryptophan synthase is an enzyme that catalyzes the final two steps in the biosynthesis of tryptophan. It is commonly found in Eubacteria, Archaebacteria, Protista, Fungi, and Plantae, but is absent from animals such as humans. Tryptophan synthase typically exists as an α-ββ-α complex.The alpha subunit is responsible for the aldol cleavage of indoleglycerol phosphate to indole and glyceraldehyde 3-phosphate: L-serine + 1-C-(indol-3-yl)glycerol 3-phosphate = L-tryptophan + D-glyceraldehyde 3-phosphate + H2O.The beta subunits catalyze the irreversible condensation of indole and serine to form tryptophan in a pyridoxal phosphate (PLP) dependent reaction. Their assembly into a complex leads to structural changes in both subunits resulting in reciprocal activation. |