Recombinant Human Cyclophilin C/PPIase C/PPIC
| Product name: | Recombinant Human Cyclophilin C/PPIase C/PPIC |
| Source: | E.coli |
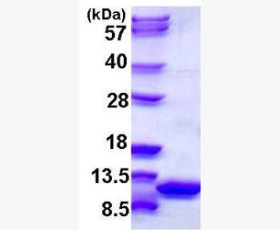
| Purity: | Greater than 95% as determined by reducing SDS-PAGE. |
| Buffer Formulation: | Supplied as a 0.2 μm filtered solution of 20mM PB, 150mM NaCl, 10% Glycerol, pH 7.4. |
| Applications: | Applications:SDS-PAGE; WB; ELISA; IP. |
| Storage: | Avoid repeated freeze/thaw cycles. Store at 2-8 oC for one month. Aliquot and store at -80 oC for 12 months. |
| UOM: | 100ug/50ug/200ug/1mg/1g |
| Source | E.coli |
| Description | Recombinant Human Cyclophilin C is produced by our E.coli expression system and the target gene encoding Lys31-Asp182 is expressed with a Trx, 6His tag at the N-terminus. |
| Names | Peptidyl-Prolyl Cis-Trans Isomerase C, PPIase C, Cyclophilin C, Rotamase C, PPIC, CYPC |
| Accession # | P45877 |
| Formulation | Supplied as a 0.2 μm filtered solution of 20mM PB, 150mM NaCl, 10% Glycerol, pH 7.4. |
| Shipping |
The product is shipped on dry ice/ice packs. |
| Storage |
Store at < -20°C, stable for 6 months after receipt. Please minimize freeze-thaw cycles. |
| Purity |
Greater than 95% as determined by reducing SDS-PAGE. |
| Endotoxin | Less than 0.1 ng/µg (1 IEU/µg) as determined by LAL test. |
| Amino Acid Sequence |
MSDKIIHLTDDSFDTDVLKADGAILVDFWAEWCGPCKMIAPILDEIADEYQGKLTVAKLNIDQNP GTAPKYGIRGIPTLLLFKNGEVAATKVGALSKGQLKEFLDANLAGSGSGHMHHHHHHSSGLVPRG SGMKETAAAKFERQHMDSPDLGTDDDDKAMAKRGPSVTAKVFFDVRIGDKDVGRIVIGLFGKVVP KTVENFVALATGEKGYGYKGSKFHRVIKDFMIQGGDITTGDGTGGVSIYGETFPDENFKLKHYGI GWVSMANAGPDTNGSQFFITLTKPTWLDGKHVVFGKVIDGMTVVHSIELQATD
|
| Background | Cyclophilin C is an enzyme (EC 5.2.1.8) found in both prokaryotes and eukaryotes that interconverts the cis and trans isomers of peptide bonds with the amino acid proline. Proline has an unusually conformationally restrained peptide bond due to its cyclic structure with its side chain bonded to its secondary amine nitrogen. Most amino acids have a strong energetic preference for the trans peptide bond conformation due to steric hindrance, but prolines unusual structure stabilizes the cis form so that both isomers are populated under biologically relevant conditions. Proteins with prolyl isomerase activity include cyclophilin, FKBPs, and parvulin, although larger proteins can also contain prolyl isomerase domains. |